Irrigated agriculture on the Central Coast is much more dependent upon groundwater than are most other farming areas of the state. The composition of well water varies quite highly throughout this region, and even wells very close to each other can have very different water compositions if they are extracting water from different aquifer formations. The chemistry of the water is of concern to the agriculturist for two main reasons; the minerals in the water can clog drip irrigation systems, and lead to undesirable changes in the quality and productivity of the soil over time.
Clogging of drip irrigation systems with mineral deposits will lead to extremely poor irrigation uniformity, and subsequently very uneven vine growth in the block. Fixing clogged emitters is extremely labor-intensive, thus proper water treatment based upon lab testing of the irrigation water is key to prevent clogging. Common mineral precipitates include lime (calcium carbonate, white colored) and iron (red-colored precipitates). Manganese, bacteria and algae can also clog systems. The white colored lime precipitates are common in this area due to the alkaline well water containing both bicarbonates and calcium. Acidification of this water with sulfuric acid or other acid material will be important to prevent precipitates from forming inside the drip irrigation system. Iron, manganese, and bacteria clogging are prevented with combinations of aeration/settling and chlorine injection; for more information see page 103 in the UC Publication "Micro-Irrigation of Trees and Vines."
The main focus of this article is on the long-term effects of our well water on soil quality for vine growth. We often do not pay as much attention to this issue because it doesn't lead to immediate problems like emitter clogging; rather the changes in soil properties resulting from the minerals in the applied water happen gradually over time, and may be imperceptible to the grower. The actual quantity of minerals applied to the soil with irrigation water can be very surprising, particularly for poorer quality waters. To appreciate the amounts of minerals that we may be applying with groundwater and their impacts on soil and vine productivity, some data taken from three wells are evaluated below.
All three wells in this example are located on the same property, but they represent a range of water quality from marginal to very poor for use in irrigating grapes. Keep in mind that grapes are relatively sensitive to salinity and boron levels as compared to many other crops; a well that might work fine for alfalfa or pasture may not be suitable at all for wine grapes.
| Total dissolved mineral content |
Well 1 |
Well 2 |
Well 3 |
|
Electrical conductivity, EC (mmhos/cm) |
1.02 |
1.59 |
2.41 |
|
Total dissolved solids, TDS (mg/l) |
639 |
997 |
1659 |
Well 1 has the lowest total mineral content, reflected in lower EC and TDS values, while Well 3 has the highest. The levels represented in Well 1 are not unusual in the area; Well 3 is much poorer quality than most (and would normally not be recommended to use for irrigating wine grapes). Both Wells 2 and 3 have elevated risk of salt accumulation in the soil if adequate leaching is not carried out. If salts are allowed to accumulate to high levels, vine growth and productivity will decline accordingly. The EC level of Well 3 is already above the thresholds of soil EC where we would expect to see declines in vine growth, hence thorough root zone leaching would be absolutely critical if using this water to any appreciable extent.
Representing the mineral content of the water on a pounds per acre-foot of water basis makes it easier to appreciate the amount of minerals that can be added to the soil with each year's irrigation.
|
Pounds of dissolved minerals per acre-foot of water (approximate amount added per acre per year in a typical vineyard) |
Well 1 |
Well 2 |
Well 3 |
|
Sodium |
245 |
525 |
979 |
|
Calcium |
158 |
98 |
112 |
|
Magnesium |
117 |
193 |
199 |
|
Bicarbonate |
620 |
979 |
1153 |
|
Chloride |
237 |
367 |
1006 |
|
Boron |
3 |
4 |
7 |
|
Total* |
1738 |
2712 |
4512 |
*Includes other minerals not shown on the list
Comparing the above numbers to the pounds of amendments and fertilizers that we typically add every season, it is easy to see just how massive the quantity of minerals is that we may be depositing on our soils with the irrigation water. Here is another way to put these numbers in perspective: for a vineyard with 6' x 10' vine spacing that receives 1 ft. of irrigation per year, Well 1 adds about 2.4 lbs of total salt per vine, and Well 3 adds about 6.2 lbs of salt per vine. The cumulative effect of many years of applications, particularly during periods of drought when very little natural leaching occurs, can lead to significant changes in the soil chemistry.
| Water pH |
Well 1 |
Well 2 |
Well 3 |
|
pH |
7.8 |
7.9 |
7.9 |
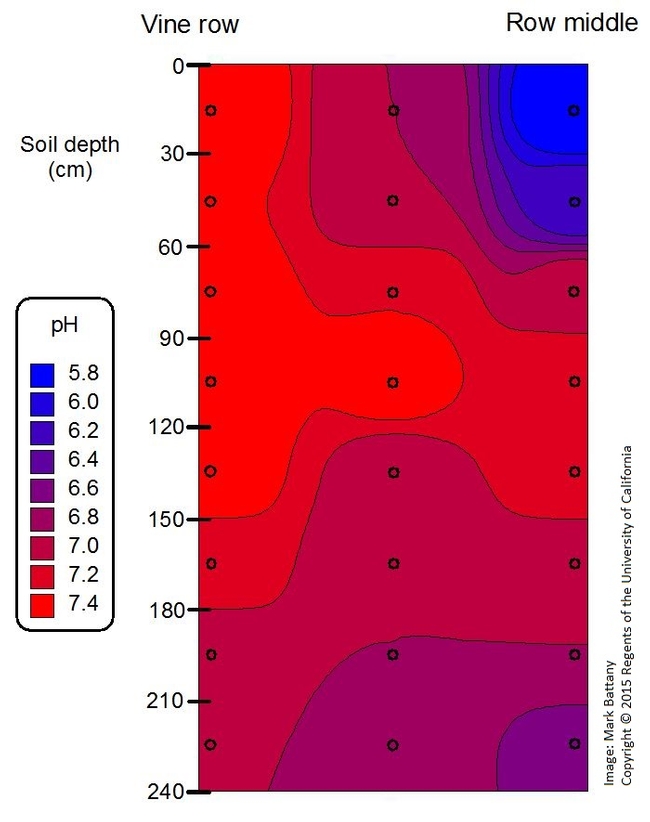
All three wells in this example have relatively alkaline water. The application of untreated alkaline water will over time increase the pH of the soil as well, particularly in the volume of soil most wetted with the irrigation water; this also happens to be the soil that has the most active roots in our dry conditions. If this soil is allowed to become excessively alkaline, it will reduce the availability of many nutrients, potentially leading to nutrient deficiencies in the vines. Thus it is important to track soil pH levels and to add amendments (acid in the irrigation system or sulfur soil amendment) to maintain soil pH in a desirable range. The diagram below shows how much the soil pH has changed in a decade-old vineyard from alkaline water applied in the vine row. The opposite condition can also occur, particularly on very sandy soils; the use of acidifying fertilizers and acid injection can lower the soil pH excessively, again creating nutrient deficiency or even toxicity problems in the excessively acidic soil.
| Boron concentration |
Well 1 |
Well 2 |
Well 3 |
|
B (ppm) |
0.98 |
1.49 |
2.62 |
Of particular interest is the level of boron, as grapes are considered to be sensitive to boron relative to many other crops. A range of damage threshold values for grapes is reported in the literature, from 0.5 ppm to 1.0 ppm. All of these three waters have elevated boron levels; Wells 2 and 3 may be completely unsuitable for grapes because of their very high boron content. Vineyards in the region having boron levels similar to Well 1 often show foliar symptoms indicating some boron toxicity; these symptoms will be aggravated if no deep leaching occurs as that allows the boron concentration in the root zone to increase over time. Boron in the soil is readily leached with water.

| Sodium Adsorption Ratio |
Well 1 |
Well 2 |
Well 3 |
|
Adjusted SAR |
2.17 |
4.27 |
7.76 |
The Sodium Adsorption Ratio (in this case the Adjusted SAR, which takes into consideration the compounding effect of carbonate/bicarbonate in the water) indicates the hazard for sodium degradation of the soil physical quality. High sodium (relative to calcium and magnesium) will dominate the soil cation exchange sites, resulting in the dispersal of the soil particles which otherwise would be aggregated in stable structures. This dispersing action reduces the soil porosity and results in a dense soil structure with poor infiltration and aeration characteristics. For Adjusted SAR values below 4.0 there is little concern about sodium hazard in sensitive crops; thus Well 1 has minimal concerns in this regard; Well 2 and more so Well 3 have some concern. These waters are better in this regard than many in the area, which often have lower calcium and magnesium levels in the water. For example, another recently drilled local well is producing water with an Adjusted SAR value of 12.3; the use of this water will lead to significant impacts on soil structure unless amended properly.
We have two methods of addressing this sodium hazard in the soil; we can add additional calcium to the soil, usually in the form of gypsum, or if the soil contains native lime (calcium carbonate) we can dissolve this lime by adding acid to the irrigation water or sulfur to the soil, and thus free up calcium that is already present. Acid is quick-acting, sulfur is slow-acting as its conversion to acid in the soil is dependent upon the activity of soil microbes. To determine which method is appropriate, the soil should be tested for the "Free Lime" content. If no lime is present in the soil, then applications of calcium amendments are the only option. Some soils may contain non-uniform levels of lime in the block; testing soil from throughout the block will be required to ascertain this. If adequate lime is available throughout the block, then acid injection with the irrigation water can be a very efficient way to correct the sodium imbalance. Regardless of how calcium is added to the soil, the rootzone will still need to be flushed with leaching water to removed the sodium that is being displaced by the added calcium. If rainfall is not sufficient to do this, then additional leaching irrigation will need to be applied.

We cannot accurately quantify water and soil chemistry conditions just by visual observation; laboratory testing of both water and soils are critical to the successful and sustainable management of a vineyard that is using marginal quality groundwater for irrigation. If labs make recommendations for practices to help maintain soil quality and vine productivity, it is important for growers to appreciate the importance of following through on these recommendations. The impacts of marginal quality irrigation water on soil health and vine productivity will be cumulative over time if not addressed adequately each year.
Additional resources:
The following FAO publication provides extremely detailed and comprehensive information on irrigation water quality:
The following series of handbooks from the UC Irrigation Program provide detailed and practical information for all aspects of operating and maintaining drip irrigation systems; they are available for purchase at the following websites:
Micro-Irrigation of Trees and Vines
Maintaining Microirrigation Systems
Agricultural Salinity and Drainage
Additional resources are available on the UCCE San Luis Obispo website: